Science of Nitrosylation and GSNOR Inhibition
GSNOR Inhibitors: Novel MOA
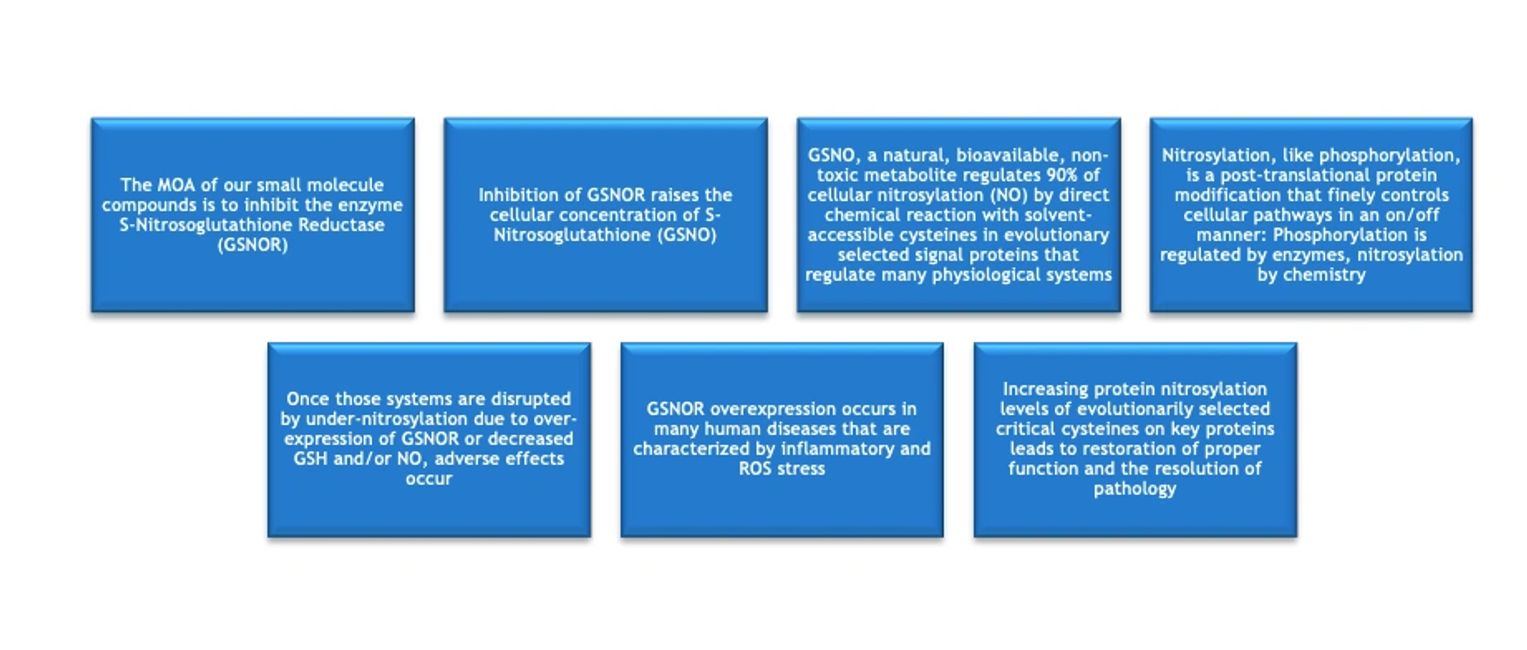
GSNOR Inhibition
GSNOR Inhibition is a Single Drug Target that Results in a Multi-mechanism Therapy for Inflammatory, Oxidant based, Mitochondrial, and Fibrotic Diseases
As Charles Darwin published more than 150 years ago in “On the Origin of Species”, evolution through natural selection has produced the amazing phenotypic diversity of living organisms on Earth. Evolution has also shaped the molecular and cellular systems that regulate the physiology of all those species. Some systems are very different—photosynthesis in plants, which is absent in animals. However, other systems, particularly signaling systems, are very similar between species. One prominent example is the nitrosylation signaling system which has been in evolution for 2-3 billion years and is important in the multi-competent cells in the hemolymph of an arthropod--the American horseshoe crab (Limulus polyphemus), an animal which has changed little over 500 million years of evolution and still depends upon its nitrosylation pathways. All other eukaryotes alive today depend upon this system as well for a diverse set of physiological responses, many of which cause disease when mis-regulated.
The importance of nitrosylation in physiology and medicine was discovered in 1977 when nitric oxide (NO), a gas, was discovered to have important signaling roles in many different cells and organs. That discovery resulted in the awarding of Nobel Prizes in 1998 to Ferid Murad, Robert F. Furchgott and Louis J. Ignarros. Dr. Murad joined SAJE’s scientific advisory board in December of 2015 and both supports and plays a key role in our programs.
Given the fact that nitrosylation, as a physiological regulator, has been conserved in evolution for so long implies that it must play important roles in maintaining organismal homeostasis or it would have been eliminated by evolution if it produced toxic consequences. Those roles are expected to be significant and diverse because evolution tends to reutilize a signaling pathway for multiple applications. In fact, if all nitrosylation from nitric oxide and GSNO were eliminated in your body, you would die within seconds. SAJE, based on its data and surveys of the relevant literature, believes that nitrosylation plays major pleiotropic roles in regulating multiple, important therapeutic pathways. To date, those expectations have been realized.
As support for these hypotheses, SAJE Pharma and its collaborators have demonstrated that inhibiting the enzyme S-nitrosoglutathione reductase (GSNOR) increases the levels of nitrosylation on critical proteins and produces numerous therapeutic benefits that are relevant to many important diseases in various organ systems. GSNOR is a reductase which breaks down or reduces S-nitrosoglutathione (GSNO) which is the stable, cellular storage form of nitrosylating activity, performing 90% of all cellular nitrosylation. GSNO has a half-life in the body of 5.5 hrs, does not break down to NO in the body, but, like NO, nitrosylates cysteines on evolutionarily selected signal and other proteins. NO, in contrast, is extremely reactive with a tissue half-life of <seconds and limited tissue penetration, whereas GSNO’s longer half-life and extensive cellular and plasma distribution make it the carrier of the major nitrosylating activities. NO reacts as an SN1 type reaction, whereas GSNO uses a different, SN2 type of chemical reaction.
Thus, by inhibiting the breakdown of GSNO by inhibiting GSNOR, the cellular levels of GSNO increase, which trans-nitrosylates by an SN2 type reaction the critical proteins that activate evolutionarily selected therapeutic pathways. SAJE Pharma’s small molecule platform regulates cellular nitrosylation, which provides, so far, 56 identified therapeutic benefits for multiple diseases with complex pathophysiological drivers, and all without any safety issues identified. Safely regulating so many disease drivers should be much more successful at benefitting complex diseases than regulating only one or a few drivers. To date, 26 animal disease models and two human diseases have been therapeutically benefited by GSNORi technology.
GSNO itself is a natural metabolite that has been shown to be non-toxic in animal studies and is freely bioavailable in blood and body tissues and can move from the intestine or other tissues into many organs including hard to access brain, and ocular spaces. GSNO is the “real” Active Pharmaceutical Ingredient (API) that does the actual therapeutic work by nitrosylating under-nitrosylated proteins in signal pathways to effect therapy. Many human diseases and animal models have increased GSNOR which can be normalized by inhibiting GSNOR. But, GSNORi is active even without GSNOR overexpression. Nitrosylation, like phosphorylation, is a post-translational protein regulatory system, but one regulated by only one enzyme, GSNOR, rather than by 700+ as with phosphorylation, so nitrosylation regulation by GSNORi is a much more druggable technology than phosphorylation regulation by so many often overlapping kinase and phosphatase targets.
Our lead molecule, SPL-850, is a composition of matter compound that we invented and own all rights to. It has no toxicity in sub-chronic safety studies or in vitro studies against 44 common targets of toxicity. SPL-850 has an unusual PK/PD relationship. It has only 0.2% oral bioavailability, yet 20-40% oral bioactivity compared to its IV bioactivity. Why? The answer is that oral SPL-850, while not systemically distributed, inhibits GSNOR in the intestine, which raises GSNO concentrations there and GSNO moves into the blood and circulates throughout the body and into many organs including the brain and other neural tissues, where it nitrosylates undernitrosylated proteins for therapeutic benefits. The intestine expresses high concentrations of GSNOR over its entire surface area, which is huge, and communicates directly with the blood circulation. The lack of systemic circulation of SPL-850, along with potent bioactivity, greatly increases the probability of clinical success, because none of the typical hazards apply that cause drugs to fail in safety or produce a low therapeutic risk/benefit equation for even marketed drugs. Because SPL-850 remains in the intestine, it will not have the following typical systemic safety issues: no first pass metabolism, no toxic metabolites, no drug-drug interactions, no inhibition of liver CypP450s, no inadequate PK, no tissue accumulation, and no off-target toxicity. The drug is active at once a day dosing in most animal disease models.
The 56 therapeutic disease benefits of GSNORis include: the Inhibition of: oxidative stress, nitrosative stress, cytokines, chemokines, inflammatory cells including microglia, TNF-, TGF-, ICAM-1, VCAM-1, NfKB, STAT3, iNOS, IL-1, NLRP3 inflammasomes, Calcium dysregulation, Mitochondrial dysregulation, NOX-4, Endoplasmic reticulum stress, Vascular dysfunction/hypoperfusion, Blood pressure in hypertensive, but not normotensive rats, Brain amyloid β, Brain Tau hyper-phosphorylation, iNOS over-expression, Nitrotyrosine expression in brain, Brain GSK 3 β and Cdk5 pathways, Brain calpain/p25/Cdk5 pathways, Hyperglycemia, Plasma glucose, Steatosis, Fibrosis, Pain, Misfolded proteins, MMP-9, BBB breakdown, and Platelet aggregation. Activation of: IL-4, IL-10, Neurotrophic factor BDNF, Neurotrophic factor CNTF, Neurotrophic factor Synaptophysin, Neurotrophic factor TrkB/pTrkB, Memory and learning, Neurological function, sGC/cGMP, Nrf-2 anti-oxidant system for ROS & RNS, and Mitochondrial prohibitin for preventing/reversing mitochondrial dysfunction. Most therapies available today and in development target only one of those drivers. Which do you think will be more effective? Please see below for the complete list.
Given this wide range of therapeutic activity, and the demonstrated activity in 26 disease models and counting, there are many clinical development options SAJE can choose. Our preference is to go for the disease(s) with the most unmet medical needs and the largest patient populations. Neurodegenerative and ophthalmic/ocular diseases are obvious choices. The 26 disease models in which GSNORis are active include: Ocular diseases, such as acute ocular inflammation, and retinal degeneration; Inflammatory and autoimmune diseases, such as rheumatoid and osteoarthritis, Crohn’s disease, ulcerative colitis, and psoriasis, etc.; Metabolic diseases, such as diabetes and non-alcoholic steatohepatitis; Cardiovascular diseases, such as acute heart attack & heart failure; Respiratory diseases, such as idiopathic pulmonary fibrosis (IPF), chronic obstructive pulmonary disease (COPD), asthma; Renal diseases, such as high salt induced chronic kidney disease and vascular dysfunction; Neurodegenerative diseases, such as traumatic brain injury and multiple sclerosis; Predicted: Alzheimer’s and Parkinson’s disease based on the inhibition of oxidant damage, inflammation, amyloid β, Brain Tau hyper-phosphorylation, and 56 total disease drivers (see above).
GSNOR inhibition increases the nitrosylation of evolutionarily selected signal transduction proteins by increasing the cellular concentration of S-nitrosoglutathione (GSNO) which trans-nitrosylates accessible regulatory cysteines. Trans-nitrosylation by GSNO proceeds by a different chemical mechanism than nitrosylation by NO. GSNO does not produce NO as some literature suggests. Furthermore, GSNOR inhibition does not cause nitrosative stress, but rather, prevents it. This lack of nitrosative stress is a clear advantage for using GSNOR inhibition rather than exogenous NO itself or non-specific NO donors to effect the therapeutic benefits of nitrosylation.
By inhibiting GSNOR, we simultaneously activate many signal pathways in a natural and balanced way to promote multi-faceted therapeutic outcomes, as noted above. This balanced approach is in contrast to drugs that inhibit or inactivate a single target that is disease related—TNF-α, TGF-β, IL-6 and many others. We believe our GSNOR inhibition approach is more efficacious and also safer because we are simultaneously regulating many disease processes in a balanced way, not obliterating a single disease driver that may also have important physiological roles. While single effect drugs, such as MABs, have therapeutic efficacy, we believe that our multi-effect drugs will be both safer and more effective for many diseases with multiple and complex pathophysiological drivers.
Some ask: How can one drug have so many therapeutic activities? The answer is that GSNOR controls ~90% of cellular nitrosylation which, like phosphorylation, is a post-translational modification that regulates many critical signal pathways, including those above. However, phosphorylation is regulated by 500+ kinases and 200+ phosphatases. Thus,phosphorylation is much less druggable than nitrosylation which is regulated primarily by GSNOR.
In summary: We believe SAJE Pharma’s data suggest that GSNOR inhibition represents a new paradigm in the pharmacological therapy of many human and animal diseases. The multiple effects that result from inhibiting GSNOR are able to simultaneously regulate multiple disease pathways in a controlled evolutionarily selected way that avoids toxicity. Thus, GSNOR inhibition by small molecules provides powerful synergistic therapies for many important diseases, with so far no identified toxicity in pre-clinical and 400 patient clinical trials.
References for all of the data discussed above:
1. Annamalai B, Won JS, Choi S, Singh I, Singh AK. Role of S-nitrosoglutathione mediated mechanisms in tau hyper-phosphorylation. Biochem Biophys Res Commun. 2015 Feb 27;458(1):214-9. doi: 10.1016/j.bbrc.2015.01.093. Epub 2015 Jan 29.PMID:25640839.
2. Anderson, C.J., Kahl A, Qian L, Stepanova, A, Starkov A, Manfredi G, Iadecola C, Zhou P. Prohibitin is a positive modulator of mitochondrial function in PC12 cells under oxidative stress. J Neurochem. 2018 Aug;146(3):235-250. doi: 10.1111/jnc.14472.
3. Artaud-Macari, E, Goven, D, Brayer, S, Hamimi, A, Besnard, V, Marchal-Somme, J, Al,i ZE, Crestan,i B, Kerdine-Romer, S, Boutten, A and Bonay, M (2013) Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxidants & redox signaling 18:66-79.
4. Assreuy, J., et al., Feedback inhibition of nitric oxide synthase activity by nitric oxide. Br J Pharmacol, 1993. 108(3): p. 833-7.
5. Balaban, Robert S., Shino Nemoto, and Toren Finkel. “Mitochondria, Oxidants, and Aging.” Cell 120, no. 4 (February 2005): 483–95.
6. Chalupowicz, D.G. and Lopez-Boada, Y. “Inhibition of S-Nitroso Glutathione Reductase (GSNOR) Up-Regulates the Nrf-2 Pathway in Human Epithelial Cells. ATSJournals.org/doi/abs/10.1164, (2013) A 1837’
7. Chen, Qiumei, Richard E. Sievers, Monika Varga, Sourabh Kharait, Daniel J. Haddad,1Aaron K. Patton,Christopher S. Delany, Sarah C. Mutka, Joan P. Blonder, Gregory P. Dubé,Gary J. Rosenthal, Matthew L. Springer. Pharmacological inhibition of S-nitrosoglutathione reductase improves endothelial vasodilatory function in rats in vivo. Appl Physiol 114: 752–760, 2013.
8. Colagiovanni, D.B., Borkhataria, D., Looker D, Schuler D, Bachmann C, Sagelsdorff P, Honarvar N, Rosenthal GJ. Preclinical 28-day inhalation toxicity assessment of s-nitrosoglutathione in beagle dogs and Wistar rats. Int J Toxicol. 2011 Oct;30(5):466-77. doi: 10.1177/1091581811412084. Epub 2011 Aug 25
9. Dubey H, Gulati K, Ray A. Amelioration by nitric oxide (NO) mimetics on neurobehavioral and biochemical changes in experimental model of Alzheimer's disease in rats. Neurotoxicology. 2018 May;66:58-65. doi: 10.1016/j.neuro.2018.03.001. Epub 2018 Mar 6.
10. Ferrini, Maria E., Bryan J. Simons, David J. P. Bassett, Matthews O. Bradley, Kevan Roberts, and Zeina Jaffar. “S-Nitrosoglutathione Reductase Inhibition Regulates Allergen-Induced Lung Inflammation and Airway Hyperreactivity.” Edited by Padraic G. Fallon. PLoS ONE 8, no. 7 (July 25, 2013): e70351.
11. Foster, Matthew W., Zhonghui Yang, David M. Gooden, J. Will Thompson, Carol H. Ball, Meredith E. Turner, Yongyong Hou, Jingbo Pi, M. Arthur Moseley, and Loretta G. Que. “Proteomic Characterization of the Cellular Response to Nitrosative Stress Mediated by S-Nitroso glutathione reductase Inhibition.” Journal of Proteome Research 11, no. 4 (April 6, 2012): 2480–91.
12. Harikesh Dubey, Kavita Gulati, Arunabha Ray. Amelioration by nitric oxide (NO) mimetics on neurobehavioral and biochemical changes in experimental model of Alzheimer’s disease in rats.Neurotoxicology, 66 (2018) 58-65.
13. Hayashida, Kei, MD, PhD Aranya Bagchi, MD, MBBS Yusuke Miyazaki, MD, PhD Shuichi Hirai, MD, PhD Divya Seth, PhD Michael G. Silverman, MD Emanuele Rezoagli, MD Eizo Marutani, MD Naohiro Mori, MD, PhD, Aurora Magliocca, MD, Xiaowen Liu, PhD, Lorenzo Berra, MD, Allyson G. Hindle, PhD, Michael W. Donnino, MD, Rajeev Malhotra, MD, MS, Matthews O. Bradley, PhD, Jonathan S. Stamler, MD, and Fumito Ichinose, MD, PhD. “Improvement in Outcomes After Cardiac Arrest and Resuscitation by Inhibition of S-Nitrosoglutathione Reductase”. Circulation. 2019;139:815–827. DOI: 10.1161/CIRCULATIONAHA.117.032488
14. Hecker, L., Vittal, R., Jones, T., Jagirdar, R., Luckhardt, T., Horowitz, J., Pennathur, S., Martinez, F., and Thannickal, V. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury Nature Medicine 15, 1077 - 1081 (2009).
15. Hee-JunNa, Hun-TaegChung, Kwon-SooHa, HansooLee, Young-GuenKwon, Timothy R.Billiar, Young-MyeongKim. Detection and Measurement for the Modification and Inactivation of Caspase by Nitrosative Stress In Vitro and In Vivo. Methods in Enzymology. Volume 441, 2008, Pages 317-327.
16. Hu, Yan-Shi, Juncai Xin, Ying Hu, Lei Zhang, and Ju Wang. Analyzing the genes related to Alzheimer’s disease via a network and pathway-based approach. Alzheimer's Research & Therapy (2017) 9:29 DOI 10.1186/s13195-017-0252-z.
17. Khan M, Shunmugavel A, Dhammu TS, Khan H, Singh I, Singh AK. Combined treatment with GSNO and CAPE accelerates functional recovery via additive antioxidant activities in a mouse model of TBI. J Neurosci Res. 2018 Dec;96(12):1900-1913. doi: 10.1002/jnr.24279. Epub 2018 Jul 19. PMID:30027580
18. Khan, M., Dammu, TS., Barrine, M., Kim., Paintlia, MK., Singh, I. and Singh AK. GSNO promotes functional recovery in experimental TBI by stabilizing HIF-1α. Behav. Brain Res. 2016 Epub ahead of print]. PMID 27780722.
19. Khan, Mushfiquddin, Bipanjeet Sekhon, Shailendra Giri, Manu Jatana, Anne G Gilg, Kamesh Ayasolla,
20. Chinnasamy Elango, Avtar K Singh, and Inderjit Singh. “S -Nitrosoglutathione Reduces Inflammation and Protects Brain against Focal Cerebral Ischemia in a Rat Model of Experimental Stroke.” Journal of Cerebral Blood Flow & Metabolism 25, no. 2 (February 2005): 177–92.
21. Khan, Mushfiquddin, Harutoshi Sakakima, Tajinder S Dhammu, Anandakumar Shunmugavel, Yeong-Bin Im, Anne G Gilg, Avtar K Singh, and Inderjit Singh. “S-Nitrosoglutathione Reduces Oxidative Injury and Promotes Mechanisms of Neurorepair Following Traumatic Brain Injury in Rats.” Journal of Neuroinflammation 8, no. 1 (2011): 78.
22. Khan, Mushfiquddin, Tajinder S. Dhammu, Harutoshi Sakakima, Anadakumar Shunmugavel, Anne G. Gilg, Avtar K. Singh, and Inderjit Singh. “The Inhibitory Effect of S-Nitrosoglutathione on Blood-Brain Barrier Disruption and Peroxynitrite Formation in a Rat Model of Experimental Stroke.” Journal of Neurochemistry 123 (November 2012): 86–97.
23. Khan, Mushfiquddin, Yeong-Bin Im, Anandakumar Shunmugavel, Anne G Gilg, Ramanpreet K Dhindsa, Avtar K Singh, and Inderjit Singh. “Administration of S-Nitrosoglutathione after Traumatic Brain Injury Protects the Neurovascular Unit and Reduces Secondary Injury in a Rat Model of Controlled Cortical Impact.” Journal of Neuroinflammation 6, no. 1 (2009): 32.
24. Langford,J., Brown AS, Wainwright RJ, de Belder AJ, Thomas MR, Smith RE, Radomski MW, Martin JF, Moncada S. Inhibition of platelet activity by S-nitrosoglutathione during coronary angioplasty. Lancet. 1994 Nov 26;344(8935):1458-60.
25. Li, Jing, Yan Zhang, Yuying Zhang, Silin Lü, Yutong Miao, Juan Yang, Shenming Huang, Xiaolong Ma, Lulu Han, Jiacheng Deng, Fangfang Fan, Bo Liu, Yong Huo, Qingbo Xu, Chang Chen, Xian Wang, Juan Feng GSNOR modulates hyperhomocysteinemia-induced T cell activation and atherosclerosis by switching Akt S-nitrosylation to phosphorylation.RedoxBiology 17 (2018) 386-399.
26. Luzina, I. G., V. Lockatell, N. W. Todd, P. Kopach, H. S. Pentikis, and S. P. Atamas. “Pharmacological In Vivo Inhibition of S-Nitrosoglutathione Reductase Attenuates Bleomycin-Induced Inflammation and Fibrosis.” Journal of Pharmacology and Experimental Therapeutics 355, no. 1 (August 25, 2015): 13–22.
27. Mao,K., Chen, s., Sun, B, et al., Nitric oxide (GSNO) suppresses NLRP3 Inflammasome activiation and protects against LPS-induced septic shock. Cell Res., 2013, 23: 201-212.
28. M. Halloran, S. Parakh, and J. D. Atkin. The Role of S-Nitrosylation and S-Glutathionylation of Protein Disulphide Isomerase in Protein Misfolding and Neurodegeneration. International Journal of Cell Biology. 2013, Article ID 797914, http://dx.doi.org/10.1155/2013/797914
29. Mishra,Sandeep,Kumar,Sonu,Singh,Shubha,Shukla,Rakesh.Shukla. Intracerebroventricular streptozotocin impairs adult neurogenesis and cognitive functions via regulating neuroinflammation and insulin signaling in adult rats. Neurochemistry International 113 (2018) 56e68.
30. Mungrue , Imran N., Robert Gros, Xiaomang You, Asif Pirani, Azar Azad, Tamas Csont, Richard Schulz, Jagdish Butany, Duncan J. Stewart, and Mansoor Husain. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J. Clin. Invest. 109:735–743 (2002). DOI:10.1172/JCI200213265.
31. Mushfiquddin Khan and Inderjit Singh. Therapeutic exploitation of the S-nitrosoglutathione/S-nitrosylation mechanism for the treatment of contusion spinal cord injury. Neural Regen Res. 2019 Jun; 14(6): 973–974. doi: 10.4103/1673-5374.250572: 10.4103/1673-5374.250572.
32. Nazem, Amir, Roman Sankowski, Michael Bacher, and Yousef Al-Abed. Rodent models of neuroinflammation for Alzheimer’s disease. Journal of Neuroinflammation (2015) 12:74 DOI 10.1186/s12974-015-0291-y
33. Olivera, Gabriela C., Xiaoyuan Ren, Suman K. Vodnala, Jun Lu, Lucia Coppo, Chaniya Leepiyasakulchai, Arne Holmgren, Krister Kristensson, and Martin E. Rottenberg. “Nitric Oxide Protects against Infection-Induced Neuroinflammation by Preserving the Stability of the Blood-Brain Barrier.” Edited by Samuel James Black. PLOS Pathogens 12, no. 2 (February 25, 2016): e1005442.
34. Ottaviani, E., Paemen, L.R., Cadet, P. and Stefano, G.B. Evidence for nitric oxide production and utilization as a bacteriocidal agent by invertebrate immunocytes (1993) Eur. J. Pharmacol 248, 319-324.
35. Paintlia MK, Paintlia AS, Singh AK, Singh I. S-Nitrosoglutathione Induces Ciliary Neurotrophic Factor Expression in Astrocytes, Which Has Implications to Protect the Central Nervous System under Pathological Conditions. J. Biol. Chem. 288(6): 3831-43, Apr. 2013. PMCID: PMID 23264628.
36. PERSPECTIVE. Streptozotocin induced Alzheimer’s disease like changes and the underlying neural degeneration and regeneration mechanism, in Neural Regeneration Research. Volume 10: 2015.
37. Pradio Kumar Kamat. Streptozotocin induced Alzheimer’s disease like changes and the underlying neural degeneration and regeneration mechanism. Neural Regen Res 2015 10: 1050-1052
38. Puzzo, Daniela, Ottavio Vitolo, Fabrizio Trinchese, Joel P. Jacob, Agostino Palmeri, and Ottavio Arancio. Amyloid-β Peptide Inhibits Activation of the Nitric Oxide/cGMP/cAMP-Responsive Element-Binding Protein Pathway during Hippocampal Synaptic Plasticity. The Journal of Neuroscience, July 20, 2005 • 25(29):6887– 6897 • 6887
39. Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Feelisch M, Fukuto J, Wink DA. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol Chem. 2004 Jan;385(1):1-10. Volume 441, 2008, Pages 317-327.
40. Rosales, Mariana; Kamila C. Silva; Diego A. Duarte; Marcelo G. de Oliveira; Gabriela F. P. de Souza; Rodrigo R. Catharino; Mônica S. Ferreira; Jose B. Lopes de Faria; Jacqueline M. Lopes de Faria. S-Nitrosoglutathione Inhibits Inducible Nitric Oxide Synthase Upregulation by Redox Posttranslational Modification in Experimental Diabetic Retinopathy. Investigative Ophthalmology & Visual Science May 2014, Vol.55, 2921-2932. doi:10.1167/iovs.13-13762.
41. Sakakima, Harutoshi , Mushfiquddin Khan1, Tajinder S Dhammu1, Anandakumar Shunmugavel1, Yoshihiro Yoshida, Inderjit Singh, and Avtar K. Singh. Stimulation of functional recovery via the mechanisms of neurorepair by S-nitrosoglutathione and motor exercise in a rat model of transient cerebral ischemia and reperfusion. Restor Neurol Neurosci. 2012 January 1; 30(5): 383–396. doi:10.3233/RNN-2012-110209.
42. Sanghani, Paresh C., Wilhelmina I. Davis, Sharry L. Fears, Scheri-Lyn Green, Lanmin Zhai, Yaoping Tang, Emil Martin, Nathan S. Bryan, and Sonal P. Sanghani. “Kinetic and Cellular Characterization of Novel Inhibitors of S -Nitrosoglutathione Reductase.” Journal of Biological Chemistry 284, no. 36 (September 4, 2009): 24354–62.
43. Saxena N, Won J, Choi S, Singh AK, Singh I. S-nitrosoglutathione reductase (GSNOR) inhibitor as an immune modulator in experimental autoimmune encephalomyelitis. Free Radic Biol Med. 2018 Jun; 121:57-68. doi: 10.1016/j.freeradbiomed.2018.04.558.
44. Taylor, B.S., L.H. Alarcon, and T.R. Billiar, Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry (Mosc), 1998. 63(7): p. 766-81.
45. Won JS, Kim J, Annamalai B, Shunmugavel A, Singh, AK, Singh I. Protective Role of S-Nitrosoglutathione (GSNO) against Cognitive Impairment in Rat Model of Chronic Cerebral Hypoperfusion, Journal of Alzheimer’s Disease. 34(3): 621-35, Mar. 2013. PMCID: PMID 23254638.
46. Zameer, Saima, Madhu Kaundal, Divya Vohora, Javed Ali, Abul Kalam Najmi, Mohd Akhtar Ameliorative effect of alendronate against intracerebroventricular streptozotocin induced alteration in neurobehavioral, neuroinflammation and biochemical parameters with emphasis on Aβ and
47. Zhang, Yuying, Kaiyuan Wu, Wenting Su, Deng-Feng Zhang, Ping Wang, Xinhua Qiao, Qin Yao, Zengqiang Yuan, Yong-Gang Yao, Guanghui Liu, Chen Zhang, Limin Liu and Chang Chen. Increased GSNOR Expression during Aging Impairs Cognitive Function and Decreases S-Nitrosation of CaMKII. The Journal of Neuroscience, October 4, 2017 • 37(40):9741–9758.
48. Zhao, Jiayi , Wei Bi, Shu Xiao, Xin Lan, Xiaofeng Cheng, Jiawei Zhang, Daxiang Lu, Wei Wei, Yanping Wang, Hongmei Li, Yongmei Fu & Lihong Zhu. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Nature Reports.e(2019) 9:5790 https://doi.org/10.1038/s41598-019-42286-8
Copyright © 2020 SAJE Pharma - All Rights Reserved.